Over the last year, labs in Kentucky, Minnesota and Switzerland made headlines with a spate of case studies. Stimulators that were originally designed to treat chronic pain have now helped about a dozen people with paralysis to wiggle their toes, flex their legs or walk with support.
This technology acts directly upon nerves; altering- or modulating- nerve activity by delivering electrical impulses to a target neurological site in the body. This is known as neuromodulation.
Since the dawn of the spinal cord injury neuromodulation era, the underlying science mantra was that the spinal cord is smart – it knows what to do, it wakes up if it gets the right sensory input, via exercise, or stimulation, or maybe a drug. Currently, spinal cord stimulation can be conducted through a non-invasive method known as transcutaneous stimulation in which stimulation of the spinal cord occurs from the surface of the skin. Additionally, epidural stimulation is an invasive technique in which a very thin lead or wire is inserted under the skin in the space just outside the spinal cord (known as the epidural space). Electrical stimulation seems to help amplify the messages being sent across the severed connection between the brain and the extremities – consequently re-establishing these links.
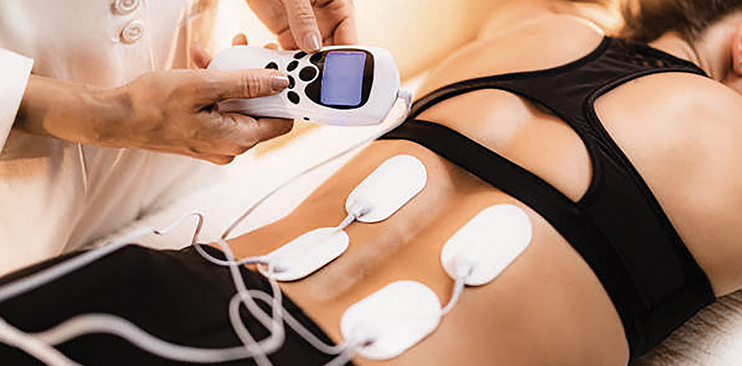
The devices also seem to offer broader benefits. Some study participants saw improvements in blood pressure, bowel and bladder control and sexual function — abilities that people with spinal cord injuries often value more than the use of their legs. In some cases, these benefits persisted even after the stimulators were turned off. The results have bolstered hopes for an improved quality of life, even for people who were paralysed years or decades ago, and the findings are upending conventional wisdom about spinal-cord injuries.
There are still major questions as to how stimulation works and why some benefits seem to persist after the stimulators are turned off. Additionally, researchers have yet to work out who might benefit the most from the procedure. The resulting improvements may not be as unanimous as researchers hope. Thus, they are looking for a way to screen individuals — because implanting a medical device inside the spine is no trivial matter. There are risks. Although the stimulators are approved by the US Food and Drug Administration to treat chronic pain, they do occasionally cause unwanted, even dangerous, side effects.
As for why some benefits persist in some participants, there are a couple of possible explanations. Stimulation might allow the individuals to participate more fully in rehabilitation, strengthening muscle and nerve connections through exercise. Alternatively, it might promote plasticity, which helps to rewire the circuits around the injury. That’s a particularly tantalizing possibility because it could mean that there’s potential for improvement over time.
This is not a cure for spinal cord injury, but it is a critical step to improve people’s quality of life.
With the further advancement of neuromodulating technology, many individuals with a spinal cord injury will be given the ability to stand, to take some steps. A cure requires biology – some regeneration of the spinal cord, possibly with cell therapies or gene modification. Some suggest that perhaps the implanted technology could be used in combination with nerve regeneration treatments somewhere down the road.
The potential of restorative therapy is filled with anticipation and hope. Clinical trials will provide the many answers we need to obtain regulatory approval, funding, and ensure safety requirements for individuals who pursue this route.
For more information on this topic, please check out this recent news article.
If you are interested in participating in research, please refer and add your information to this platform.
Contributors: Sam Maddox,
Cassandra Willyard, Gabriela Ocampo and Barry Munro